2.1 Ocular Micrometer
Introduction
Ocular micrometer is a glass disk that fits in a microscope eyepiece and that has
a ruled scale. In some
microscopes, the ocular has to be disassembled so that the disk can be placed
on a shelf in the ocular tube between the two lenses. However, in most of the
microscopes, the ocular micrometer is simply inserted into the bottom of the
ocular. Before an ocular micrometer can
be used, it is necessary to calibrate it for each of the objectives by using a
stage micrometer. The physical
length of the marks on the scale depends on the degree of magnification. When
calibrated with a stage micrometer, direct measurements of a microscopic object
can be made. To conclude it, ocular micrometer can be used to measure the size
of magnified object. It can also be used to compare the size of prokaryotic and
even eukaryotic microorganisms. When the ocular micrometer is placed in the
eyepiece, the line superimposed certain distance markers on the microscope
field. The distance between
the lines of an ocular micrometer is an arbitrary measurement that only has
meaning if the ocular micrometer is calibrated for the objective being
used. A stage micrometer, also known as
an objective micrometer, has scribed lines on it that are exactly 0.01mm (10
micrometers) apart. The exact distance between each ocular division measures on the microscopic field can be
calculated by determining how many units of the ocular micrometer superimpose a
certain distance on the stage micrometer. The calibration is important in order
to obtain the measurement with more
accurate and precise. In addition, It is important to know that the system
should be recalibrated when the objective lens is changed. After calibration of
the ocular micrometer, the stage micrometer is replaced with a slide containing
microorganism. The dimension of the microorganism used, including length and
width will be determined based on the calibration of system done before.
Objective
1.)
To learn the
proper way of measuring and counting cells using a microscope
2.)
To learn the
technique in calibration of the ocular micrometer
Materials and Reagents
Microscope fitted with an
ocular micrometer
Slide micrometer
Stained preparation of yeast
and bacteria
Procedure
1.)
The stage
micrometer is placed on the stage
2.)
The microscope
is focused using the lowest power objective until the image on the stage
micrometer is observed superimposed on the eyepiece scale.
3.)
The amount of
the divisions of the eyepiece scale corresponding top a definite number of
divisions on the stage scale is determined.
4.)
The measurement
of an eyepiece division in micrometer is calculated.
5.)
The process is
repeated by using the high-power and oil immersion objective.
6.)
An example is
shown as below:-
Each division of the stage micrometer = 10 µm.
If 100 eyepiece divisions = 11 stage division = 110 µm,
then :
1 eyepiece division = 110/100 = 1.1 µm
7.
The diameter of the field for each objective is calculated and recorded for
further reference.
8. The average
dimensions of a sample of yeast cells is determined and the process is repeated
using a sample of bacterial cells.
 |
| Ocular Micrometer |
 |
| Stage Micrometer |
.jpg) |
| Stage Micrometer |
The figures below shows the
calibration and the calculation of the measurement of the samples. First image
shows the superimposed image of the ocular micrometer and the stage micrometer.
The ratio of the two
micrometers are evaluate by simple calculation:
Taking point A as 7.8 units
and point B as 11.6 unit sin the scale of ocular micrometer, we are able to
conclude that:
10 division in the stage
micrometer (equivalent to 0.1 mm) = (11.6-7.8) = 3.8
After getting the ratio of the scale of ocular
micrometer to the scale of the stage micrometer. We are now done with the
calibration part and will begin our measuring procedure for the specimens.
5 specimens which are closer to the ocular scale are
chosen due to the reason of accuracy of the readings.
The calculation are carried out by using the ratio of
0.1 mm is to 3.8 units to calculate the real measurement of these samples. The
average reading will be calculate and taken as the final result.
The average = The total of stage measurement / the total number of specimen
= 394.74 / 2
=
78.948
= ( two decimal places )78.95µm
2.2 Neubauer Hemocytometer Chamber
Introduction
For microbiology, cell culture, and many
applications that require use of suspensions of cells, cell concentration is
necessary to be determined and identified. One can often determine cell density
of a suspension spectrophotometrically, however that form of determination does
not allow an assessment of cell viability, nor can one distinguish cell types.Counting chamber is a device used for determining the number of cells per unit volume of a suspension. The most widely used type of chamber is called a hemocytometer, since it was originally designed for performing blood cell counts. The hemocytometer was invented by Louis-Charles Malassez and consists of a thick glass microscope slide with a rectangular indentation that creates a chamber. This chamber is engraved with a laser-etched grid of perpendicular lines. The device is carefully crafted so that the area bounded by the lines is known, and the depth of the chamber is also known. It is therefore possible to count the number of cells or particles in a specific volume of fluid, and thereby calculate the concentration of cells in the fluid overall.
 |
| Neubauer hemocytometer chamber |
Materials
and Reagents
Yeast culture
Neubauer and coverslip
Sterile dropper
Procedure
1. The
empty neubauer hemocytometer chamber is observed under the microscope with 40x
objective lens.
2. The
coverslip is placed on the H-shaped trough.
3. By
using the sterile dropper, the yeast culture is transferred into the trough of
the empty neubauer hemocytometer carefully to avoid the formation of air
bubbles.
4. The
neubauer with the yeast culture is observed under the microscope again with the
same magnification.
5. The
number of yeast cells in the 16 randomly chosen squares are recorded.
Counting
1. The
large middle square of the neubauer hemocytometer is chosen.
2. The
16 smaller squares are randomly chosen from the large square.
3. The
number of yeast cells is counted from the 16 small squares.
4. The
average number of yeast cells per small squares is calculated (Only the cells
inside a square and the cells that touch the upper and left grids are
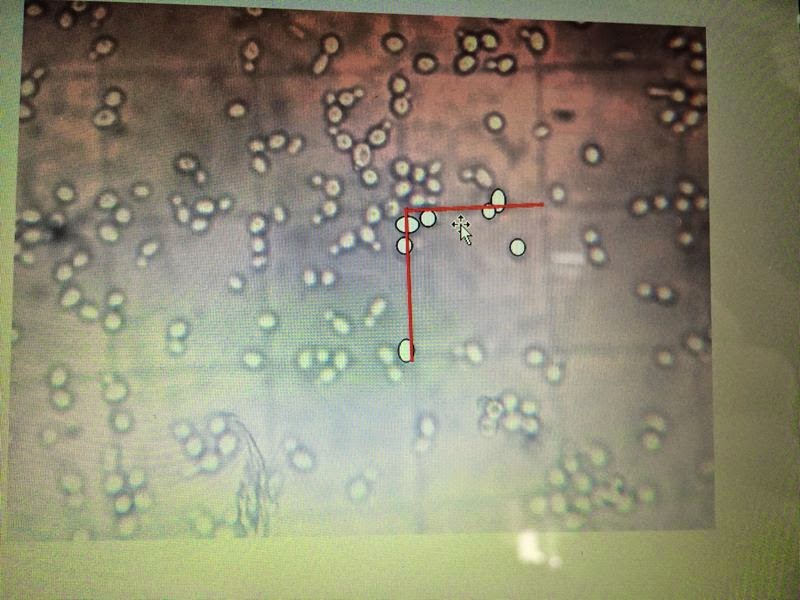
counted. For example, there are 7 yeast
cells counted in a small square with red grids on the top and left in the diagram below.)
5. The
volume confined in a small square is calculated.
6. The
cell concentration per ml is calculated using the average number of yeast cells
and the volume confined in a small square.
Results
1. Data
of number of yeast cells in the 16 small squares:
18
|
14
|
10
|
8
|
16
|
7
|
13
|
10
|
12
|
2
|
14
|
15
|
16
|
4
|
8
|
7
|
Total = 174 cells
Average/ Mean = 174/16 = 10.875 cells
per small square
2. Length
of small square = 0.05 mm
Depth
of the small square = 0.1 mm
Volume
confined of a small square = 0.05 x 0.05 x 0.1= 2.5 x 10-4 mm3
3. Average
number of yeast cells in 16 squares per volume confined of the square = 10.875
/ 2.5 x 10-4 mm3 =
43500 cells/ mm3
4. Number
of cells in 1 cm3 of yeast culture = 43500 / (0.1)3
= 43500000 cells/ cm3
5. Since
1 ml = 1cm3,
number of cells in 1ml yeast culture = 43500000 cells/ ml
Conclusion
With ocular micrometer, we are able to
measure the size of the specimen more precisely. With the nuebauer
hemocytometer chamber, we are able to count the number of cells more accurately
and are able to determine the cell concentration in a culture.
Reference





ReplyDeleteLab Automation